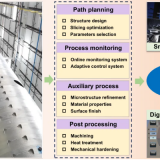
干细胞具有发展成人体所有类型的细胞的能力,或者是通过引导来分化成特定的细胞类型,为生产类器官奠定,并且通过3D打印技术制造的干细跑培养支架,所培养的生物组织可以用于药物开发和个性化的药物测试。
最近,牛津大学的研究人员发现了一种高精度的3D打印干细胞培养人体组织的方法,该人工组织可用作药物毒性测试,科学家将能够看到某些药物成分如何与人体组织反应,从而减少对动物实验的需要。
事实上,3D生物打印人体组织一旦发展成熟,不仅仅可用于药物毒性测试,还是修复人体受损区域的一种手段。
牛津大学的这一生物3D打印技术发表在“科学报告”杂志的一篇研究论文中,牛津大学(化学系、生理学、解剖与遗传学系)和布里斯托大学(分子医学中心)的跨学科团队共同合作,从而找到了一个新的通过3D打印干细胞来培养人体和动物组织的方法。
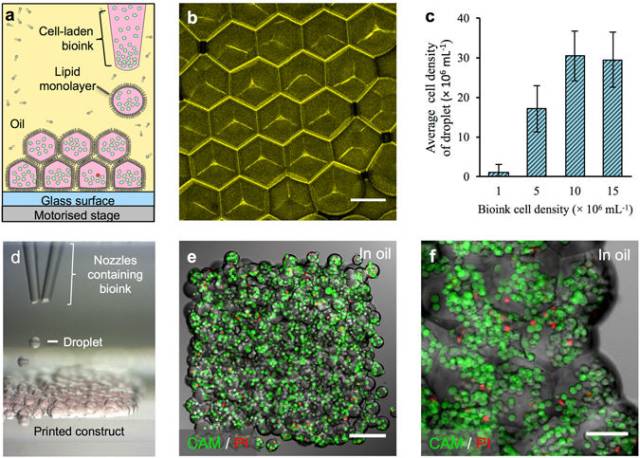
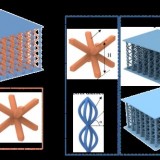
3D printing of cellular constructs. (a) Schematic of cell printing. The dispensing nozzle ejects cell-containing bioink droplets into a lipid-containing oil. The droplets are positioned by the programmed movement of the oil container. The droplets cohere through the formation of droplet interface lipid bilayers. (b) A confocal fluorescence micrograph showing droplet interface bilayers (stained yellow) within a cell-free printed construct (11 × 14 × 7 droplets). The bilayers were visualised by adding sulforhodamine-101 (~10 μM) to the print solution. (Scale bar = 100 μm). (c) Histogram showing the mean HEK-293T cell density in printed droplets under oil as a function of the cell density in the bioink. The cell density was calculated as the mean number of cells per droplet (n = 25) divided by the mean droplet volume. Error bars represent the compound error of droplet size variance and cell per droplet variance. (d) A bright-field micrograph of a patterned cell junction, containing two cell types, printed as successive layers of 1 nL droplets (d = 130 μm) ejected from two glass nozzles (d = ~150 μm). (e) A confocal fluorescence micrograph of a printed HEK-293T cellular construct (11 × 14 × 2 droplets) under oil. Live/dead cell staining was performed with calcein-AM (CAM, green) and propidium iodide (PI, red), respectively. Visible are approximately 700 cells at 4 × 107 cells mL−1 with a viability of 85% (determined by manual cell counting). (Scale bar = 150 μm). (f) A high magnification, confocal fluorescence micrograph of a live/dead assay performed on an HEK-293T cellular construct (7 × 8 × 4 droplets) printed at a starting concentration of 1.5 × 107 cells mL−1, with a mean occupancy of 38 cells per droplet equivalent to 3 × 107 cells mL−1. Visible are some of the droplet boundaries. (Scale bar = 75 μm).
这种打印技术被成为“液滴”3D打印技术,在牛津化学系化学生物学教授Hagan Bayley的带领下获得了成功培养复杂组织的成果。“液滴”3D打印技术的原理是将活性组织细胞包裹在脂质涂层中,这种方法可以提高单个细胞的存活率。OxSyBio(牛津合成生物学)的主要作者和生物科学家Alexander Graham博士解释说:“我们的目标是制造具备天然生物体的基本行为和生理学的三维生物组织。 我们将相对便宜的组件组装成高分辨率的细胞3D打印设备,用来制造具有一定复杂性的人造组织。”
High-resolution patterning of two cell types. (a–c, e,f) Confocal fluorescence micrographs of printed cellular constructs in oil, immediately after printing. HEK-293 cells stained with Deep Red (DR) or Red CMPTX (RC) CellTracker™ dyes were false-coloured blue and yellow, respectively. (a) A Y-shaped structure within a square construct (8 × 9 × 4 droplets), with a mean feature width of 180 μm. (Scale bar = 200 μm). (b) A cruciform pattern of HEK-293 cells within a square construct (10 × 12 × 5 droplets). (Scale bar = 250 μm). (c) A high magnification image of the patterned HEK-293 cells in (b). (Scale bar = 100 μm). (d) A 3D model of a cuboidal cellular construct with an interface between two HEK populations (HEK 1, yellow; and HEK 2, cyan) at a diagonal in the x-z plane. (e,f) Partial cross-sections at fixed vertical positions (45 and 192 µm respectively) of a cellular construct (21 × 24 × 7 droplets) printed based according to the model in (d), showing both HEK populations. (Scale bars = 250 μm). (g–j) Side-on images of lamellar constructs, comprising CellTracker™ stained HEK-293 cells before and after phase transfer. The lower, DR-stained HEK-293 cell layers (yellow) were 3 droplets thick, while, the upper, RC-stained HEK-293 cell layers (blue) were 4 droplets thick (g,h) or 3 droplets thick (i,j). Images were recorded: (g) at day 0, in oil, immediately after printing; (h) immediately after transfer to culture medium; (i) on day 3 of culture, in medium and; (j) on day 5 of culture, in medium. (Scale bar = 250 μm).
研究团队希望其新的3D打印方法可以对世界各地的医疗保健产生积极影响,从而消除动物实验的需要,以及推进组织再生疗法的进程。
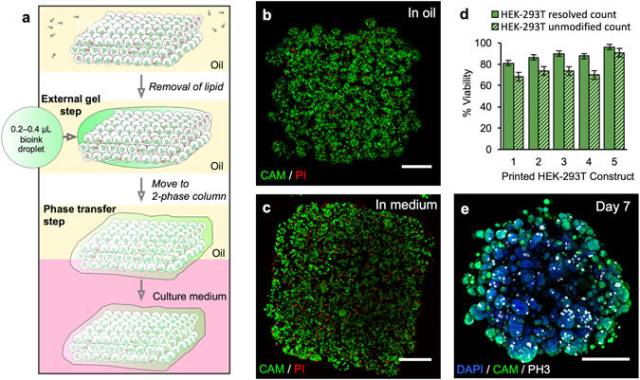
Phase transfer and culture of printed constructs containing HEK-293T cells. (a) Gel encapsulation of a printed construct and phase transfer. The printed cellular construct was gelled by standing at 4 °C for 20 to 25 min and the lipid in the oil was removed by washes with silicone oil AR20 at room temperature. The construct was then coated with a thin layer of cell-free bioink, which was gelled by standing at 4 °C for 20 to 25 min. The gelled construct was then transferred into the upper phase of an oil-culture medium two-phase system. The construct fell through the oil into the culture medium. (b) Image of a z-stack 3D reconstruction of live/dead-stained HEK-293T cells printed as a cuboid construct (7 × 8 × 4 droplets) immediately after printing under oil. The printed droplets had a mean density of 2.9 × 107 cells mL−1 with a viability of 96%. (Scale bar = 200 μm). (c) Image of a z-stack 3D reconstruction of live/dead-stained printed HEK-293T cells after gel encapsulation and transfer to culture medium. (Scale bar = 200 μm). (d) Graph showing HEK-293T cell viability (including standard error of the mean) of five printed constructs at day 0 after transfer to culture medium. Viabilities were determined by using automated object counting, values of which were used either unmodified or resolved with respect to mean cell size. (e) Image of a z-stack 3D reconstruction of immunocytochemistry performed on a construct in culture medium at day 7: cell nuclei (DAPI, blue); cytoplasm of live cells (CAM, green) and; mitotic marker (phospho-histone H3 ICC, PH3, white). (Scale bar = 200 μm).
鉴于3D打印技术的潜在影响,研究人员已经注意到技术的商业化前景。于是在2016年1月,从Bayley实验室中孵化出伦敦医疗3D打印公司OxSyBio这家初创企业,这家公司将寻求“工业和生物医学目的”的新技术商业化。
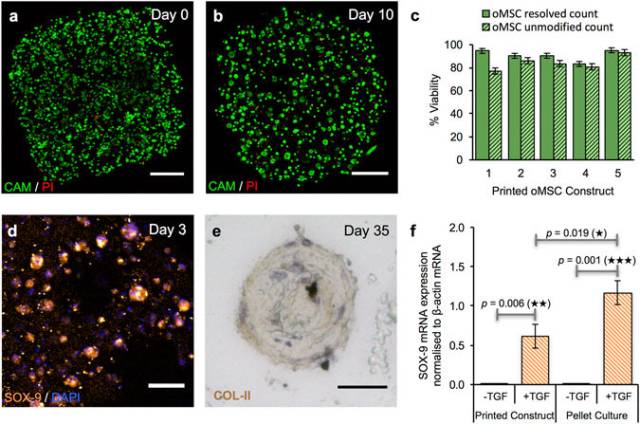
Growth and differentiation of printed oMSCs. (a,b) Image of a z-stack 3D reconstruction of live/dead-stained printed oMSCs: (a) immediately after printing and; (b) after 10 days in culture with the TGF-β3 supplement. (Scale bars = 250 μm). (c) Graph of oMSC viabilities (including standard error of the mean) for five printed constructs immediately after transfer to culture medium. Viabilities were determined by using automated object counting, values of which were used either unmodified or resolved with respect to mean cell size. (d) Confocal fluorescence micrograph of immunocytochemistry performed on a printed oMSC construct after 3 days of culture with a TGF-β3 supplement: SOX-9 (orange); nuclei (DAPI, blue); cytoplasm of live cells (CAM, calcein-AM, green). (Scale bar = 50 μm). (e) High-magnification micrograph of immunohistochemistry performed on a printed oMSC construct after 35 days of culture with TGF-β3 supplement; type II collagen (diaminobenzidine tetrahydrochloride (DAB), brown); nuclei (hematoxylin QS, blue). (Scale bar = 25 μm). (f) Digital PCR measurements of SOX-9 mRNA expression in printed oMSC constructs (n = 22) and oMSC pellet cultures (n = 24) after 7 days in chondrogenic medium with or without supplementation of TGF-β3. Each printed and pellet sample was replicated 4 to 6 times from four oMSCs sources, each extracted from a different sheep. SOX-9 expression was normalised to an endogenous β-actin control. Error bars represent standard deviations. Differences were tested by using a paired t-test, with two-tailed p values < 0.05 considered significant.
OxSyBio首席技术官Sam Olof博士说:“生物3D打印有许多潜在应用,我们相信可以通过使用来自患者的细胞来模拟或增强天然组织功能来创建个性化治疗。 3D打印的生物组织也可用于诊断应用 – 例如用于药物或毒素筛选。”
下载资料,请加入3D科学谷3D产业链QQ群:529965687
查找往期文章,请登陆www.51shape.com,在首页搜索关键词
网站投稿请发送至editor@51shape.com